Extracting flavour series: Breaking down how supercritical fluid extraction works

In the fourth instalment of our series on extracting flavour, head distiller and co-founder of Hepple Spirits Company Chris Garden explains how Hepple uses supercritical fluid extraction to extract juniper oil.
Supercritical fluid extraction is used in many industries, from the fragrance industry through to the pharmaceutical industry. By applying high pressure and temperature to a fluid in a controlled environment, such as a chamber, you can force the fluid to have the properties of both a liquid and a gas at exactly the same moment. This supercritical fluid will have a density similar to liquid and a viscosity and diffusion rate similar to a gas.
A supercritical fluid performs as an extremely good solvent and achieves a high extraction efficiency and yield of the desired flavour components. Many different fluids can be used to achieve these extractions from carbon dioxide to propylene and the selection of which supercritical fluid depends on the substance you wish to extract. At the Hepple Spirits Company, we use carbon dioxide as it has a moderate critical temperature and pressure to achieve supercritical fluid status and it can easily be separated from the extract as it is a gas at room temperature, and it works as an extremely good solvent when extracting juniper oil.

Getting started
It has taken a few years to perfect our production process but now we have a consistent method for the extraction of juniper oil from the organic Bulgarian juniper that we import. We start with 800g of juniper that is ground up in a powerful food processor for 10 minutes.
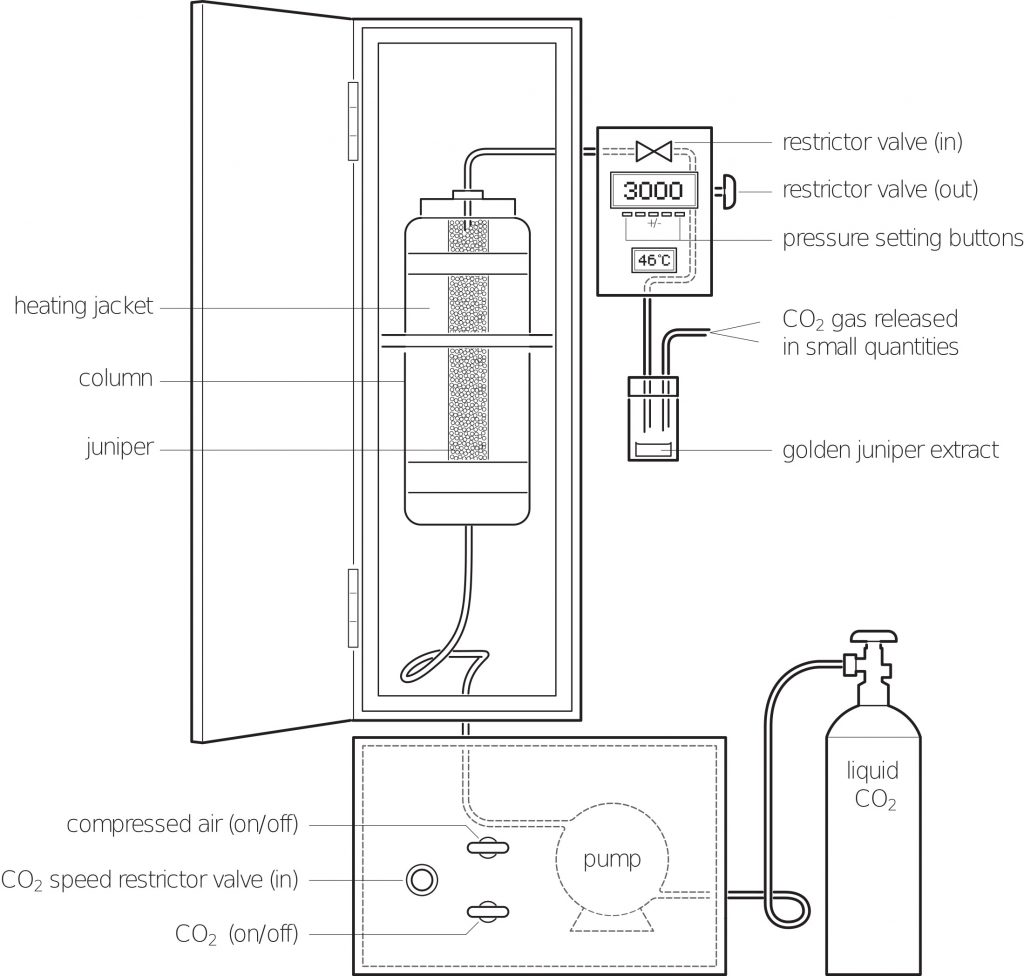
This reduces the berries to a sticky coarse powder and increases the surface area that the supercritical fluid will be exposed to, thus making the extraction of the juniper oil easier. The mound of ground up juniper is transferred to the column where the removed lid is reaffixed and the exit valve is firmly shut. It should be noted that the column must be thoroughly cleaned between extractions as the smallest piece of juniper can cause a leak in the seal and this allows the carbon dioxide to escape the column meaning it is impossible to achieve the desired pressure.

What happens next?
Once the lid is reaffixed and the exit valve shut the carbon dioxide cylinder is opened and the carbon dioxide is allowed to reach the piston pump in the base of the supercritical extraction unit. The pump is turned on and as the pump sends more carbon dioxide into the column, the pressure increases. While this is happening the heating jacket around the column is set to 40OC.
As pressure and temperature are related, i.e. an increase in temperature leads to an increase in pressure, the unit must be monitored and the pressure adjusted so that once the 40OC is achieved in the heating jacket the pressure in the column is 3,000 PSI. This pressure is the equivalent to going to just over 2,000m below sea level.
Once the supercritical fluid has been achieved the exit valve is opened a small fraction and the supercritical fluid moves through the ground up juniper where the juniper oil dissolves into the carbon dioxide and is taken down a tiny pipe and into a collection vessel. As the collection vessel is at room temperature the supercritical fluid returns to a gas and is separated from the juniper oil. From the original 800g of juniper we are left with 15ml of juniper oil.
What we get
This oil is super concentrated and can be used in the production of just over 1,000 bottles of Hepple gin. A little goes a long way and it would be foolish to use too much of the oil as it would overpower the other flavours that are present in the gin and obtained through copper pot distillation and vacuum distillation.
This super-concentrated extract contains flavour molecules that cannot be obtained through any form of distillation, it also contains a much higher quantity of these flavour molecules. For example, the extract contains 11 more sesquiterpenes than the copper-pot-distilled juniper equivalent – these are the flavour components that give a woody and piney flavour to the gin, the extract also contains five times as much D-limonene which gives citrus flavours to the gin.
Overall, the supercritically extracted juniper oil achieves the closest flavour profile to juniper other than juniper itself.